
For our June Member Spotlight we talked to Ensign Pharmaceutical, a Nebraska-based startup company on a mission to develop and market novel pharmaceutical products for the treatment of osteoarthritis and related musculoskeletal disorders.
Tell us about Ensign Pharmaceutical.
Ensign Pharmaceutical is a Nebraska-based start-up company. Ensign Pharmaceutical’s mission is to discover, develop and market novel pharmaceutical products that free patients from pain and disability associated with musculoskeletal disorders and inflammatory diseases while avoiding potential damaging side effects and assisting in more effective healing.
Ensign received a SBIR fast track award of $1.93 million in May 2020 from the National Institute of Drug Abuse to support the preclinical development of ProGel technology. The company also received a $200,000 matching grant from the Nebraska Department of Economic Development. Ensign leased premium lab space from the University of Nebraska Medical Center College of Pharmacy for the development and commercialization of ProGel products.
What is the technology platform behind Ensign Pharmaceutical?
ProGel technology has multiple innovative features, making it optimally suited for commercial development. Any number of pharmaceuticals can be combined with ProGel, making it a “platform technology.” Platform technologies like ProGel can be used for localized and sustained delivery of a variety of therapeutic agents to treat a broad spectrum of clinical conditions.
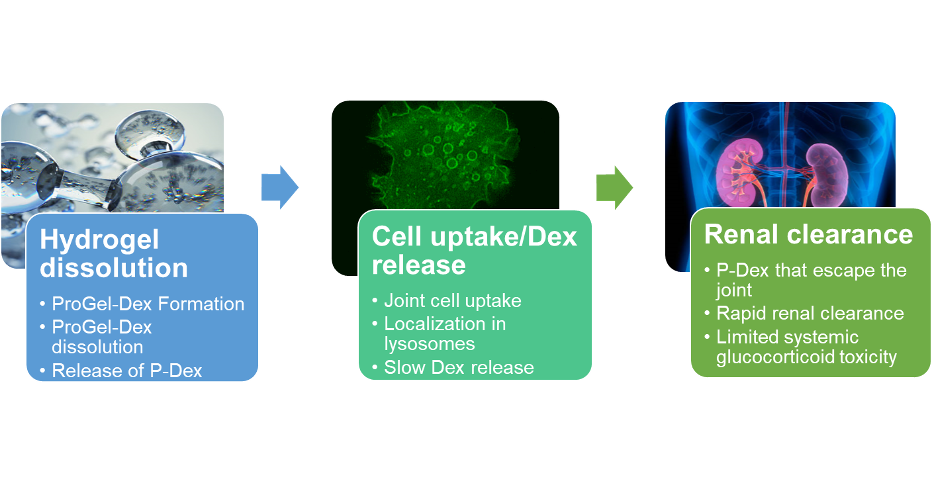
Ensign’s first product incorporated a potent steroid, dexamethasone, into the ProGel formulation. ProGel-Dex is formulated for injection into arthritic joints. At room temperature ProGel-Dex exists as a liquid which is ideally suited for intra-articular injection. Inside the joint it transforms into a gel-like substance as it reaches body temperature. The gel is then retained in the affected area, concentrating the pharmaceutical payload exactly where it needs to be. The unique capacity of ProGel-Dex to provide continuous active Dex release results in sustained suppression of inflammation and associated pain, overcoming the limitations of current steroid formulations that have been shown to be rapidly cleared from the joint, which accounts for their relatively short duration of action. Due to the low molecular weight of the prodrug (~6 kDa), the prodrug released from the joint space is swiftly cleared by the kidney, which ensures low systemic steroid exposure and reduced potential systemic for steroid toxicity.
Who are the key members of the Ensign Pharmaceutical team?

What are some of the biggest challenges facing your industry today and how does Ensign Pharmaceutical play a role in helping overcome those challenges?
Osteoarthritis (OA) is the most common form of arthritis, affecting more than 30 million individuals in the United States. OA is characterized by progressive loss of articular cartilage and alterations in peri-articular bone that are associated with chronic pain, inflammation, and loss of mobility. Currently, no treatment has been shown to alter the progression of joint structural damage or promote repair in patients with OA. Effective joint pain control is the primary objective of management of the disease. Osteoarthritis Research Society International (OARSI) guidelines recommend intra-articular steroids as an appropriate treatment for all OA patient subgroups. The utility of steroids for long-term pain management has been hampered by the short half-life in the joint and resultant short duration of the anti-inflammatory and analgesic effects. Therefore, there remains an unmet clinical need for an effective and safe intra-articular steroid therapy to provide sustained pain relief for clinical management of OA.
Ensign’s ProGel-Dex technology addresses this critical issue by providing sustained pain and inflammation relief while avoiding adverse side effects. With its unique safety and efficacy, ProGel-Dex is a strong and viable drug candidate for the clinical management of OA pain with a straightforward pathway for regulatory approval and translation into clinical use in patients suffering from the disabling symptoms of OA.
Why is Nebraska an important location for your company?
ProGel technology was invented at the University of Nebraska Medical Center with Dr. Wang, Dr. Goldring and Dr. Zhao as leading inventors. Rheumatology and orthopedic programs at UNMC are world-class and have established the Nebraska Arthritis Outcomes Research Center, which has the personnel and expertise to conduct the clinical trials that will provide the definitive evidence of the efficacy and safety of this novel therapy for OA and fulfill the regulatory requirements for translation of ProGel-Dex into an effective therapy for OA pain and inflammation.
